- cross-posted to:
- technology@lemmy.ml
- cross-posted to:
- technology@lemmy.ml
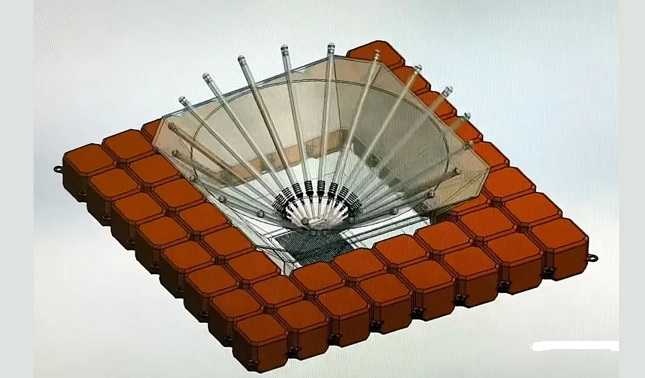
Offers better brine handling and produces higher-purity water, making it ideal for offshore green hydrogen production. Sustainable and efficient solution with low environmental impact.
It’s not clear from the article, but if this is a direct solar-to-dessalination I can understand how it uses less energy (why does it use any energy at all?) than other methods with pumps and filters, the issue is rate maybe, but I can’t find a paper about this.
Found https://fuelcellsworks.com/news/malaga-students-patent-an-innovative-solar-desalination-system-to-produce-green-hydrogen/ which says it produces 1 cubic meter per day, which is great for small-scale seaside production. Again, I have no access to details of how 1 square meter of sunlight (or more, maybe they use mirrors to concentrate sunlight, it says 9 square meters, as kerfuffle mentioned) can dessalinate 1 cubic meter of water per day, but it’s great if it works, just wondering why solar dessalination hadn’t been tried to this degree of success before.
there are nearly thousands of ideas ,research, for deselinase water but its always had problem that it is not cheaper than current system or its not cheaper to maintain . In capitalism our first prirority with any breaktrough is that it should be more profitable.
Typically people will prefer the option that gives them a $50 water bill over a $200 one.
water should be free
How do we cover the expense of extracting and purifying it?
tax ?
Okay, so I’m a government that has collected $1 billion in taxes, do I choose the solution that provides water to 1 million people or 2 million people at the same cost.
Having a price tag isn’t just a feature of capitalism, it represents effort and labor. Even if we didn’t have money, we would be deciding if we should spend our villagers’ time building the water purification system that provides water to half the village or the one that provides to the whole village.
deleted by creator
deleted by creator
Current techniques appear to use around 1.8-2kWh/m3 for desalination. That would imply that this uses only 350-400Wh. You’d think you could her better production from 9m2 just going up a few solar panels and putting a conventional plant. I presume the author has no fucking clue how desalination of solar heating and distillation works and/or hasn’t even read the patent to find out how this works.
Good on the student team for thinking creatively, but they seem to have only two engineers but four managers on the team. And not a single coherent description from those four managers, who seem very proud of taking credit for the students’ work.
I’m wondering if the “amazing” part is that its just physically smaller? It says less brine which means either its not pumping through a ton of seawater…or some how making the salt go somewhere. So I’m thinking its that its a small foot print and cheap materials meaning that its accessible to more people.
From some digging the “amazing” part is that it’s floating. I’m not sure how they do distribution and collection, but from the UMA page it’s a vapor distillation process - same as the old survival distillation process but on a floating platform. Solar energy heats the water, evaporating some, and it is passed over a cooler to condense the water. The “no brine” claim comes from the fact that they so inefficient that the localize increase in brine concentration due to the extraction of desalinated water is within the limits for not requiring treatment - they’re just lifting evaporate from the surface and (I’m guessing) using a low power circulation loop to draw water from below the surface as their condensation cooling loop. I suppose it’s possible that the cooling may even be a convectively driven vs a photoelectric conversion and standard pump, which would be useful for low maintenance. It’s a cool project, no doubt. I’m still not sure how they’re doing bulk collection and distribution of 1000kg of water a day. Maybe that’s the 17% energy figure. Maybe it’s someone else’s problem to solve.
I appreciate that you dug this up. That’s actually sort of cool little project.
The problem with the old condensation technique (other then the fact that it is naturally inefficient) is that depending on where you are and air currents you can’t always depend on a great output when humidity drops. This solves that problem as the ocean is constantly evaporating. But that also means that this is just a project that can’t go much further, as you can’t really scale it for global use. There is a finite amount of humidity in the air, and absorbing it from one place just reduces it in another. You would be better off just floating giant rain collectors into the ocean… at least then you’re only stealing water that would have became salinated anyway.
Thanks for digging
A single student’s project used 17% of a power plant’s energy production??
That’s Insane! What the hell was he doing? Training chatGPT 7 on a supercomputer??
I hope you’re not being serious lol. The article says the desalination plant designed by this student uses 17% of the power a normal desalination plant, meaning a 5+x reduction in energy consumption.
I know you’re just trying to get me to read the article instead of commenting on the headline like I know what it’s all about, but that’s not going to happen!
The article is meh on details (other than to disclose it was a team and that they want to focus this on green hydrogen).
I gotcha… someone else dug up the UMA paper and it’s a condensation system. So nothing new really, other then it being floating in the ocean. Maybe something new… they said that paper was also light on some details.
The article says the desalination plant designed by this student uses 17% of the power a normal desalination plant, meaning a 5+x reduction in energy consumption.
“With nine square meters, it consumes only 17% of energy compared to traditional desalination plants.”
Comparing based on size doesn’t seem too useful. How many square meters is a “traditional desalination plant”? How much salty water can it purify into drinkable water given a certain amount of energy compared to the student’s design?
I hope it’s an improvement over existing designs, but unfortunately this article doesn’t have any actual content. It’s clickbait that hopes people will jump to conclusions like “it’s a 5x reduction in energy compared to the traditional approach” because that drives traffic.
About as much clickbait as the team’s titles. I wonder if they called in the senior hygiene engineer after someone took a big dump in the toilet:
María Martínez leads the engineering team, Pedro Benito is responsible for information technology systems, Ignacio Colombo is the director of operations, Juanjo Vallejo is the executive director, and Ayman Bouachi and Andrés Florido are the engineer’s mechanical and electrical, respectively. Alejandro Rodríguez is the director of the School of Engineering and the mentor of this project at UMA.
It’s a student project in which they’re probably supposed to be approaching it from a business perspective anyway.
In which cases they’re pretty much normal titles. “Director of” is usually a non profit title while the equivalents in this case would be “COO (Chief Operations Officer)” for director of operations. Executive director would be CEO. And then you have usual VP level titles with IT Director and Engineering Director. And then you have two engineers, a mechanical engineer and electrical engineer.
Mining crypto?
Green hydrogen?
What other colors can it come in?
No seriously… Isn’t all hydrogen “green?” Is there bad hydrogen? 🤔
Green/grey/blue/whatever refers to the production process and the energy sources used to make the hydrogen, because the production and powering it with electricity also requires emitting greenhouse gases. Most of the hydrogen produced today is gray hydrogen produced using fossil fuels. I don’t understand why we hear so much about hydrogen as being the fuel of the future, when straight electrification would be more efficient, but maybe I’m missing something.
For some things hydrogen doesn’t really make sense, but for everything else it’s basically a form of a battery. The one big advantage it has over batteries is its weight.
Is there bad hydrogen? 🤔
The Hindenburg would have some things to say about this…
To be fair, it wasn’t designed for hydrogen, but helium.
It’s like putting diesel into a petrol engine (or vice versa), and saying petrol is bad when the engine inevitably explodes.
Yup. Green hydrogen comes from splitting water with electricity from renewables.
Other flavours of hydrogen come from splitting natural gas, using electricity from burning stuff. Which isn’t as green.
How do you handle the brine? “Minimal amounts” of brine is not an answer. What’s a minimal amount? What are you going to do with it? If you dump it back in the ocean, you increase salinity.
I can’t find a better explanation anywhere for how it works than “it uses condensation”
So I have to guess the seawater is evaportated directly from the waters surface or something, which would just leave all the salts in there. That would be exactly as problematic as a traditional desalination plant pumping it’s brine back into the ocean. Maybe my guess about how it works is wrong though.
Yeah, it seems to me that you only have three options when it comes to the brine, and none of them are good- bury it and hope it doesn’t get into the water table, leave it where it is and hope it doesn’t leak, or dump it back in the ocean and increase ocean salinity. I don’t see another option.
I wonder how expensive shipping brine would be… Probably very until we can get it ludicrously concentrated.
But then I imagine we could take it somewhere like the middle of an uninhabitably hot desert and just fill up a low point in the topology. The combination of distance from where people live and dry hot conditions drying it out as fast as possible would possibly work?
It wouldn’t be worth it unless shipping the brine was cheaper than just shipping clean water from places that have it to places that don’t. But who knows.
The brine solution will contain concentrated amounts of elements, like lithium. That concentrated solution may be economicly viable to process.
Fine. What do you do about the salt?
Fine. What do you do about the salt?
We distribute it to fanbases across the internet, who are adept at managing it.
Most media requires a lot of salt to give to their users, we’ll never run out of demand for it
deleted by creator
Why is increasing salinity of the ocean bad? Doesn’t that happen all the time on a much greater scale as ocean water evaporates into the atmosphere?
Your link (or at least what I’ve read from it) doesn’t cover why it would be bad to make the ocean saltier; it only talks about the rise of the salty ocean water.
I think it makes it pretty clear how increased salinity affects the climate, but here you go-
https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2021GL095748
I’m not suggesting that it’s not bad, but that article didn’t seem to talk about rising ocean salinity.
I don’t know if that works with brine. But what I know is that many regions fill old mines with salt and heat it up with excess energy from solar in the summer to use as general energy storage and for heat in the winter. That might be a good way to repurpose the brine
I said this in another comment- I’d be very worried it would get into the water table. That brine is not just salt. It’s also full of toxins. But maybe there are good solutions that I don’t know about.
Yeah it was just something I came up with. We can only hope that someone will find a good solution on what to do with it
This is very good news if it can be scaled.
Come back in 3+ years according to the article